Menu
CLINICAL GRADE CELL LINES
FEEDER DEPENDENT (FD)
| CELL LINE # | HAD-C 100 PCB (MCB) | HAD-C 100 2 CB (WCB) | HAD-C 102 PCB (MCB) | HAD-C 106 PCB (MCB) | |||
|---|---|---|---|---|---|---|---|
| TYPE | hESCs | hESCs | hESCs | hESCs | |||
| SEX | Male | Male | Male | Male | |||
| hPSCReg ID # | HADe007-A | HADe007-C | HADe008-A | HADe009-A | |||
| NIH APPROVAL # | NIHhESC-11-0123 | NIHhESC-11-0123 | NIHhESC-11-0124 | NIHhESC-11-0125 | |||
| ASSOCIATED PUBLICATION | Tannenbaum SE et al., Derivation of Xeno-Free and GMP-Grade Human Embryonic Stem Cells – Platforms for Future Clinical Applications. June 20, 2012. PLoS One | ||||||
| RELATED PRODUCTS | ●HAD-C 100 Matched Research-grade Bank (RG); ●HAD-C 100 Feeder-Free (FF) Seed Cell Bank;●HAD-C 100 WCB Feeder-Dependent (FD) Cell Line; | ●HAD-C 100 PCB Feeder-Dependent (FD) Cell Line; | ●HAD-C 102 Matched Research-grade Bank; ●HAD-C 102 Feeder-Free (FF) Seed Cell Bank; | ●HAD-C 106 Matched Research-grade Bank (RG); ●HAD-C 106 Feeder-Free (FF) Seed Cell Bank; | |||
| CULTURE PLATFORM | CellGro; Umbilical Cord Feeders | CellGro; Umbilical Cord Feeders | CellGro; Umbilical Cord Feeders | CellGro; Umbilical Cord Feeders | |||
| PASSAGE # | P11 | P16 | P20 | P24 | |||
| PASSAGING METHOD | Manual Passaging | Manual Passaging | Manual Passaging | Manual Passaging | |||
| CELLS FROZEN AS | Cell Clusters | Cell Clusters | Cell Clusters | Cell Clusters | |||
| DOCUMENTATION AVAILABLE | Embryo Donor Testing Results | ||||||
| QC Characterization Results | |||||||
| Safety Testing Results (Hy Laboratories, Rehovot--Israel; Bioreliance--UK if available) | |||||||
| Quality System Statements for GMP Facility (Hadassah, Israel) | |||||||
| GMP Certificate for Hadassah GMP Facility (Ministry of Health, Israel) | |||||||
| List of Hadassah hESC Research Center QA SOPs for hESC Derivation, Culture and Banking | |||||||
| List of Raw Materials (catalogue and lot numbers) for the Generation, Maintenance and Banking | |||||||
| Brief Summary of the Donor Medical History | |||||||
| Information About the Embryo (gamete donation) for the relevant HAD line | |||||||
| Information About the Cytogenetic Stability of line | |||||||
| Certificate of Analysis for the Relevant HAD line | |||||||
| Ethics Committee Documents from IRB | |||||||
| Additional Documentation Available as Necessary | |||||||
| Images |
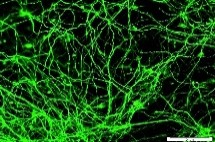 HAD-C 100 1O CB
HAD-C 100 1O CB β-TUBULIN |
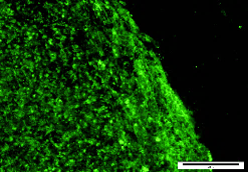 HAD-C 100 2O CB
HAD-C 100 2O CB SSEA3 |
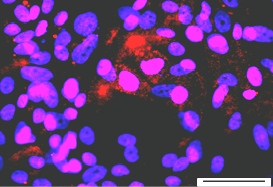 HAD-C 102 1O CB
HAD-C 102 1O CB m-actin/DAPI |
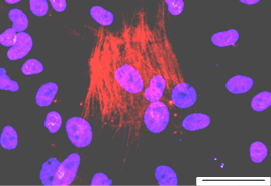 HAD-C 106 1O CB
HAD-C 106 1O CB SOX-17/DAPI |
|||
